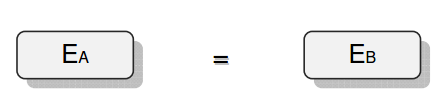|
(c) 2012 - 2017 * updated MAR 2020 |
||||||||||||||||||||||||
 |
||||||||||||||||||||||||
 |
||||||||||||||||||||||||
 |
||||||||||||||||||||||||
 |
||||||||||||||||||||||||
|
|
|
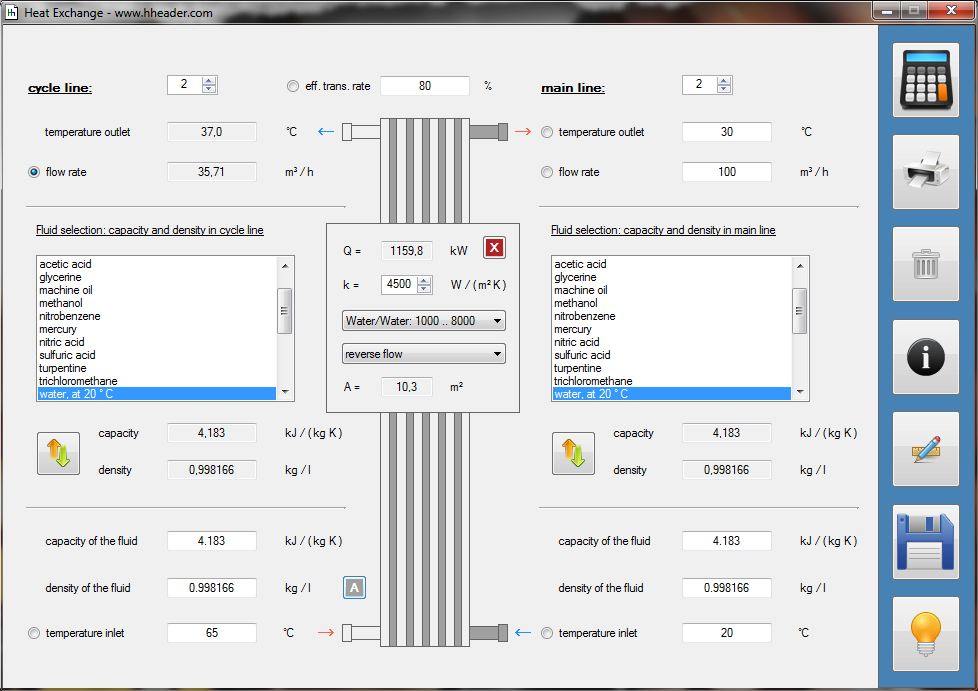
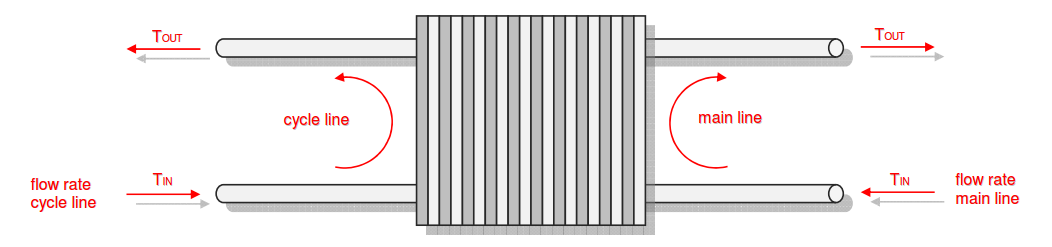
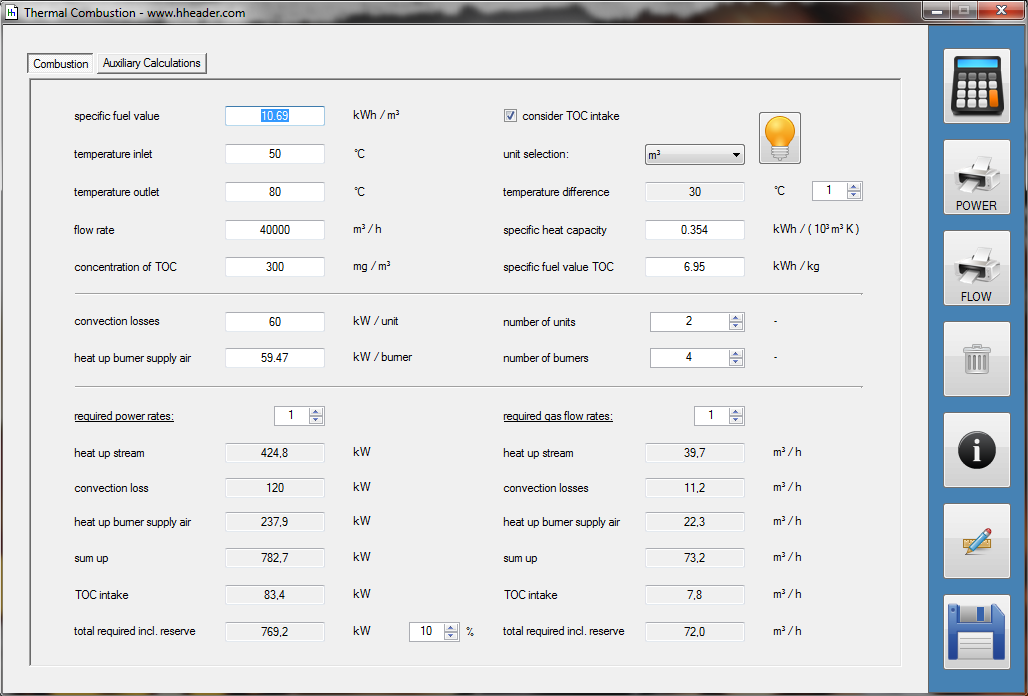
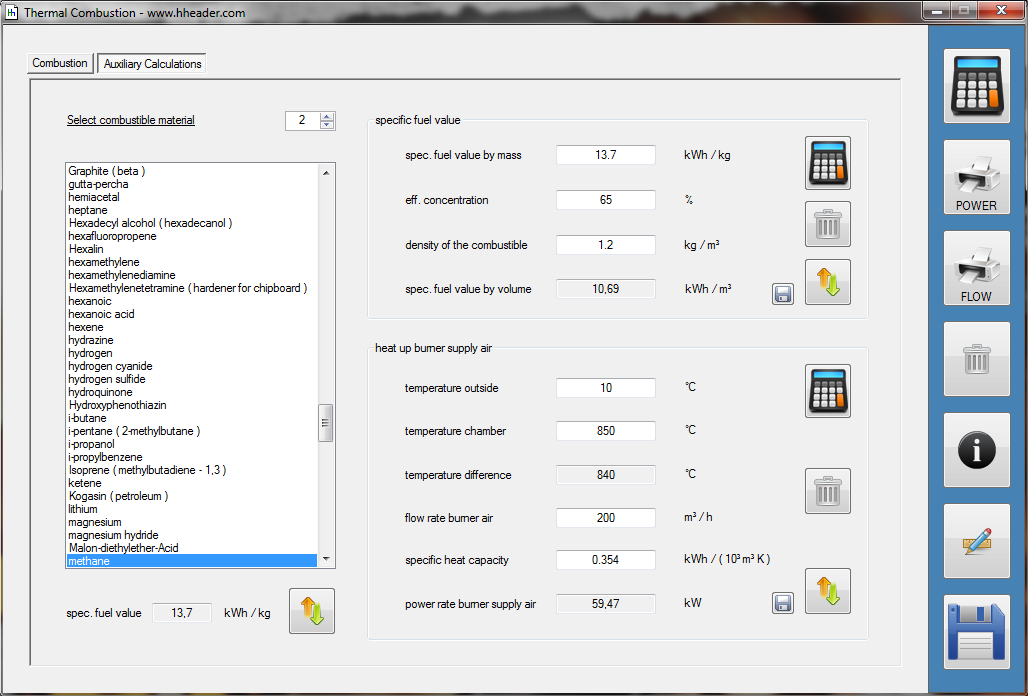
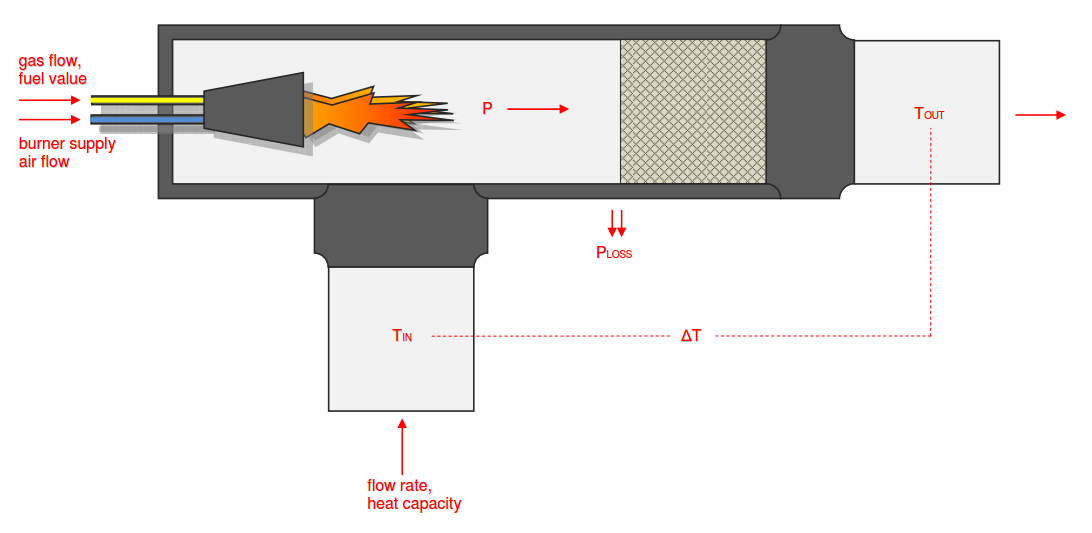
Category 4 - Energy: Thermal CombustionCombustion chambers can be built and designed for many different use cases. At least and in every case some kind of fuel or combustible is required. No matter what kind of combustion chamber and no matter which combustible is used, the energy balance is always an interesting part for these types of plants, especially if burners are used for increasing the temperature of an airflow or mass-flow. Finally the focus is on the demand of the used combustible, which is resulting out of the energy balance. Auxiliary calculations for the specific fuel value of the combustible and the power rate that is required for the heat up of the burner supply air are also provided. |
|||||||||||||||
|
|
|||||||||||||||
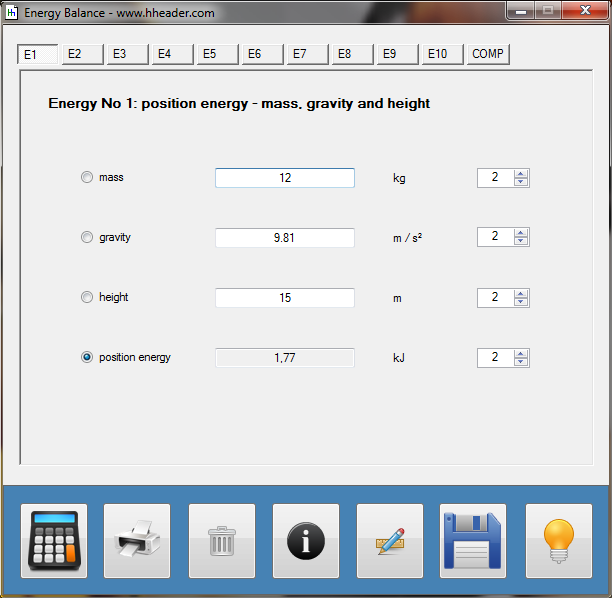
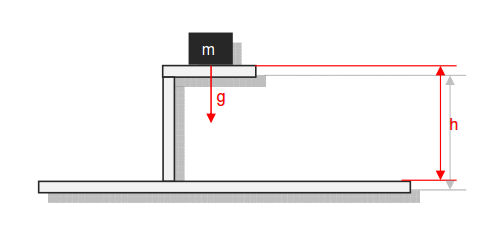
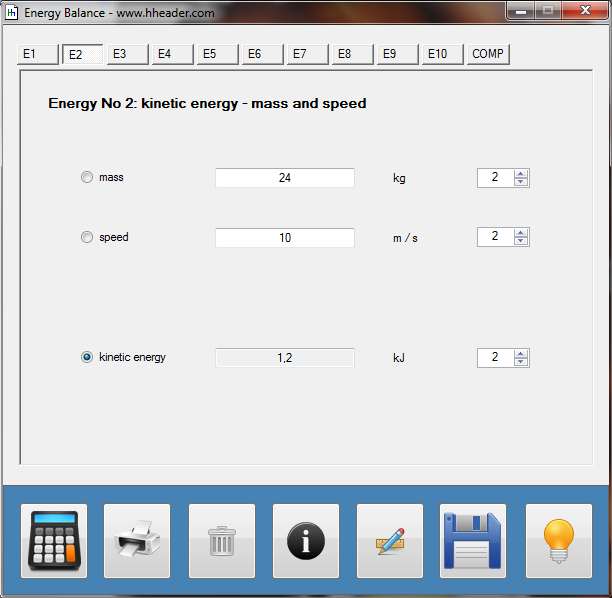
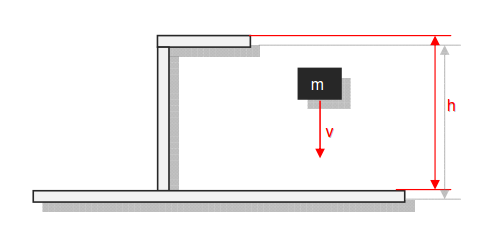
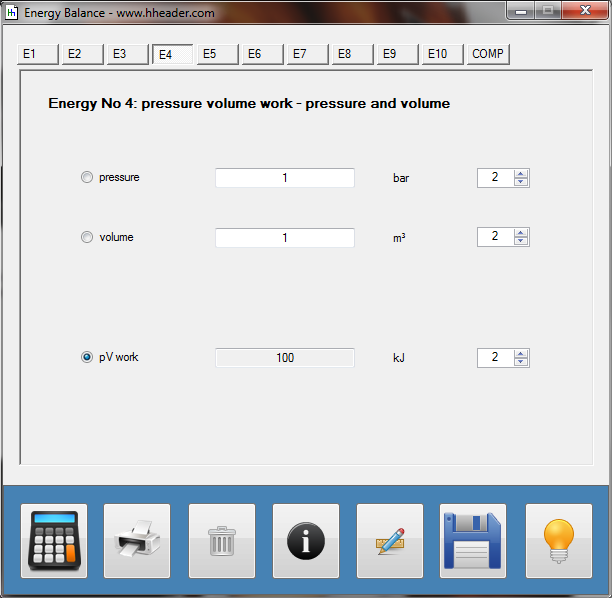
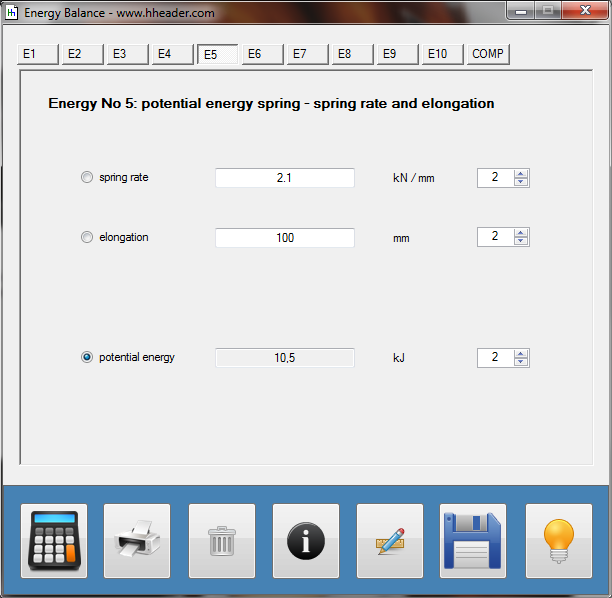
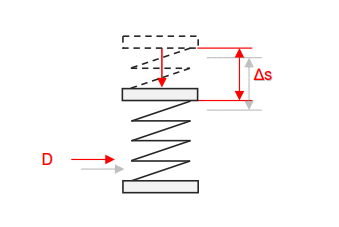
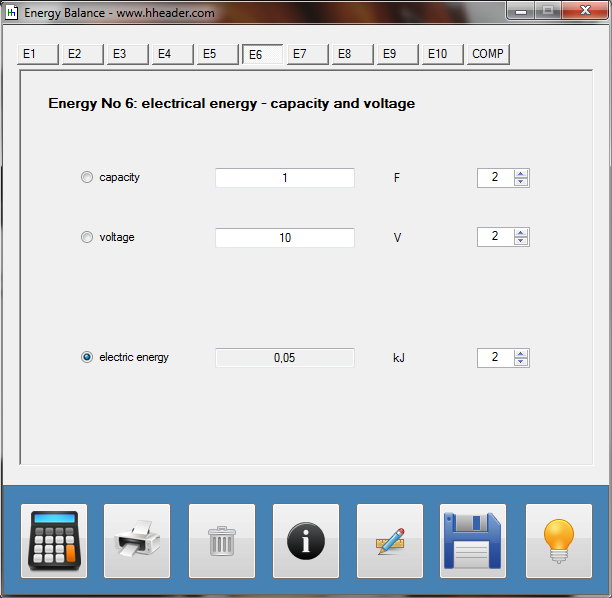
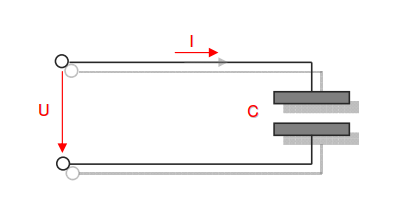
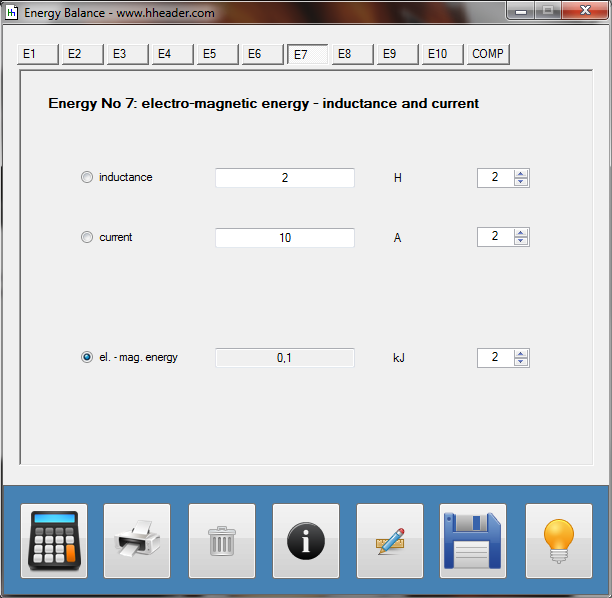
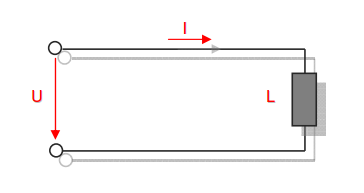
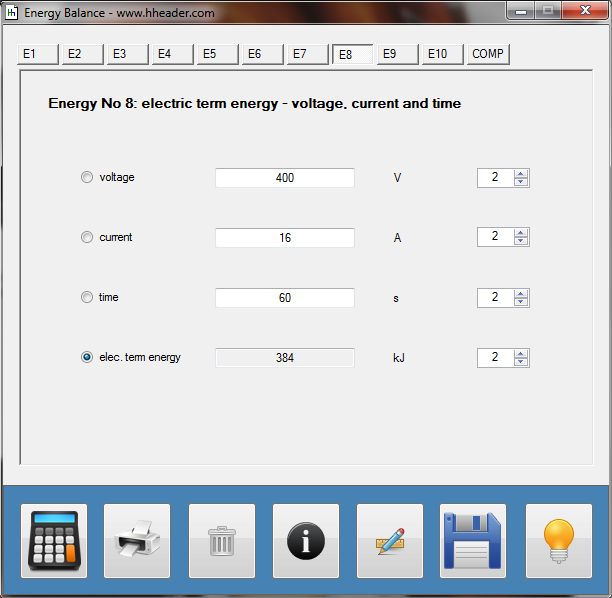
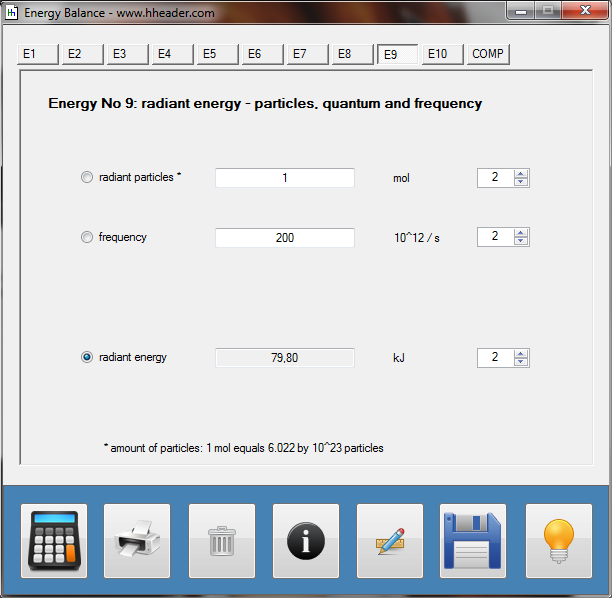
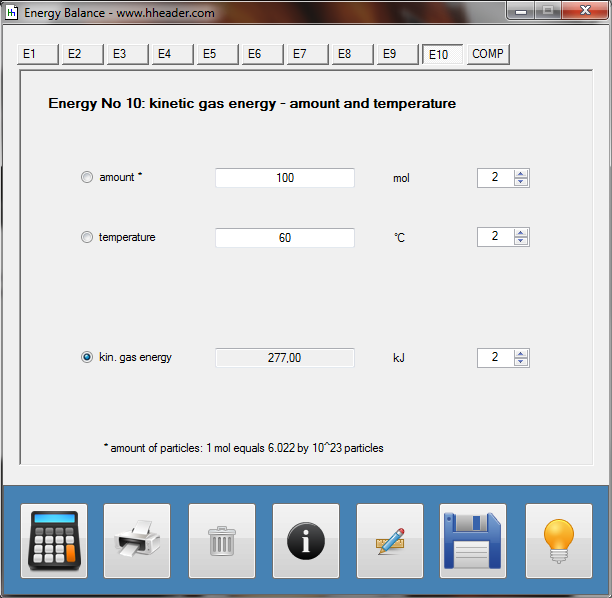
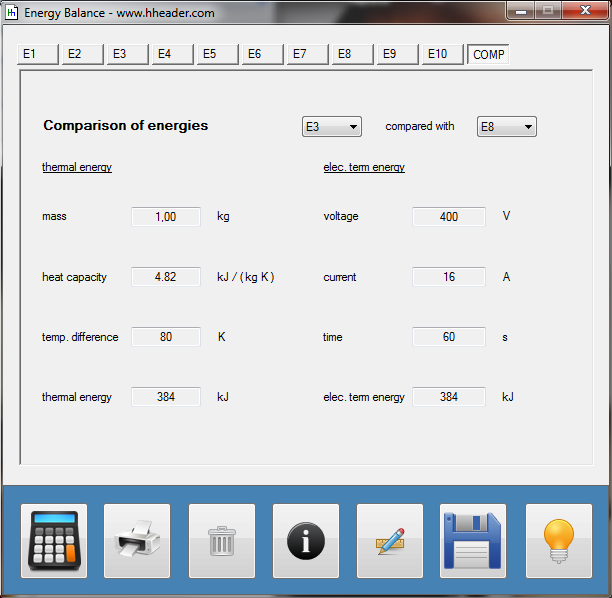
Category 4 - Energy: Energy BalanceEnergy cannot get lost - energy can be converted from one kind to another kind. There are many different kinds of energy. Energy can appear, can be stored and can be converted. Some of these kinds, which are often relevant for technical processes, are: position energy, kinetic energy, thermal energy, pressure volume work, potential energy spring, electrical capacitive energy, electro-magnetic energy, electric term energy, radiant energy, kinetic gas energy. The program provides the corresponding calculations for the mentioned energy kinds. The law of conservation of energy is a powerful tool to evaluate technical or process circumstances. |
|||||||||||||||
|
|
|||||||||||||||
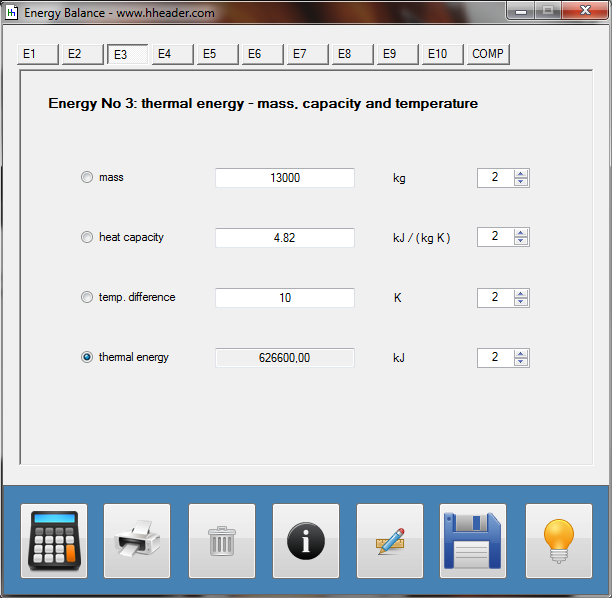
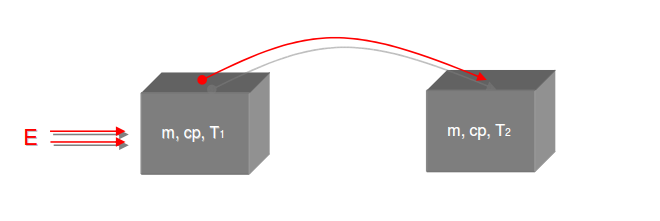
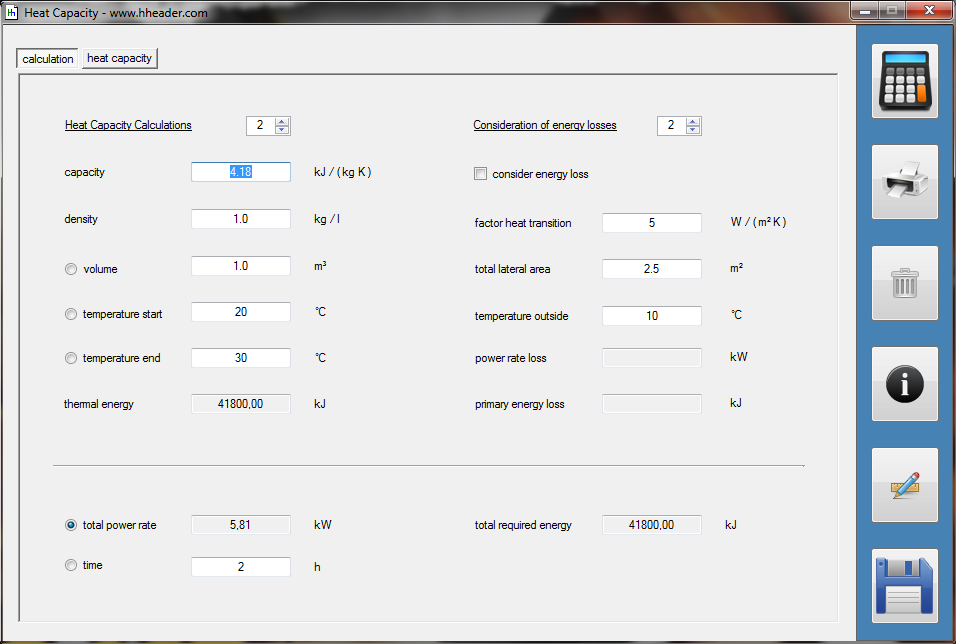
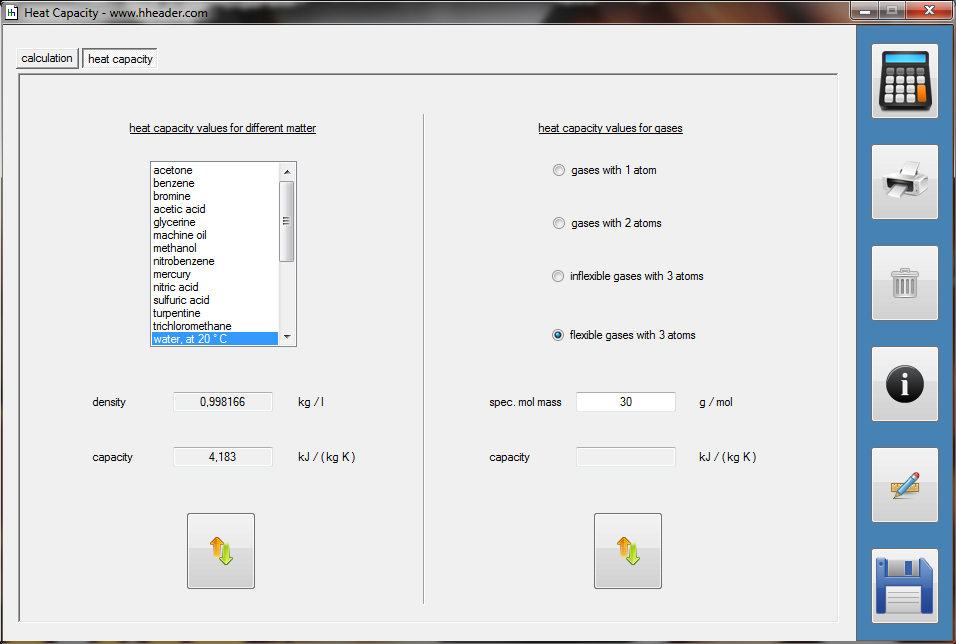
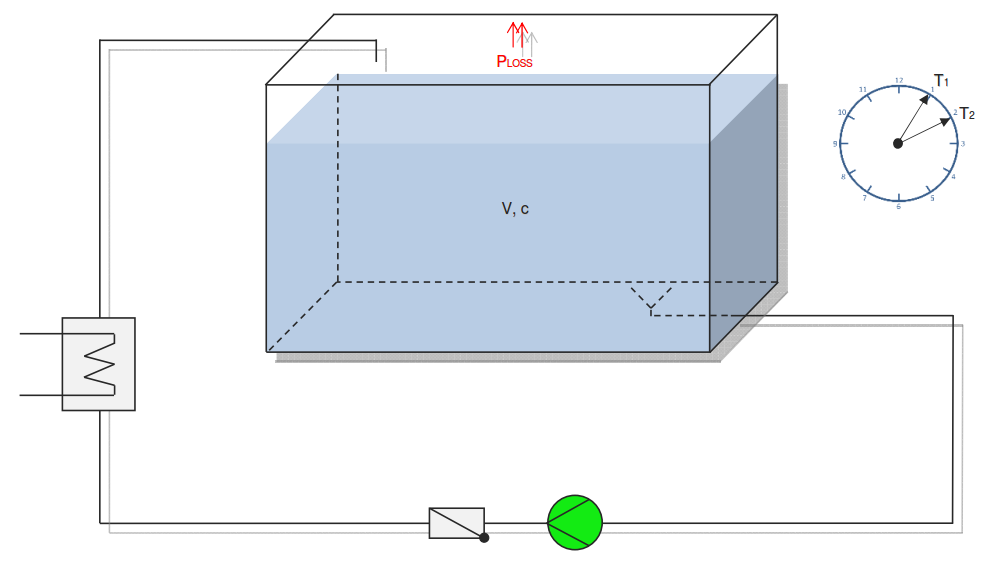
Category 4 - Energy: Heat CapacityPart of many processes is a direct or indirect heating up of different fluids. That means at least a defined volume of a fluid with specified heat capacity and density is heated up from a start temperature to an end temperature. Therefore the temperature difference is relevant. Regarding this it is important to know which amount of thermal energy is required for increasing the temperature, how long this procedure will last, and which power rate is required, accordingly. In practice on site there will be power losses like convectional losses beside others. This even if the system (piping, tanks and compounds) has a proper insulation. Anyway the program provides a selection by which the consideration of energy losses can be activated or deactivated. |
|||||||||||||||
|
|
|||||||||||||||
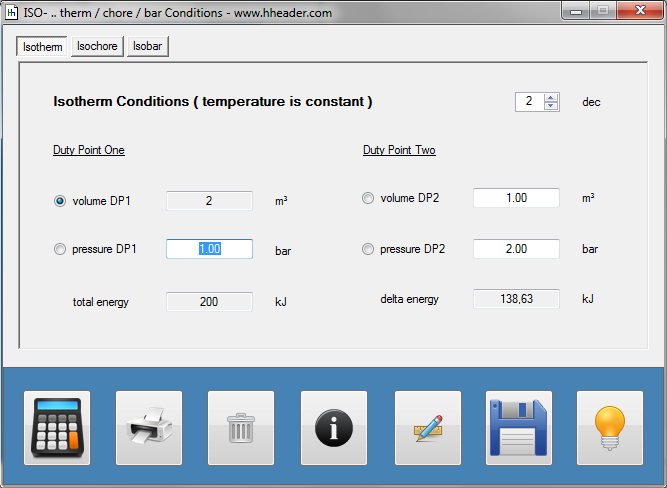
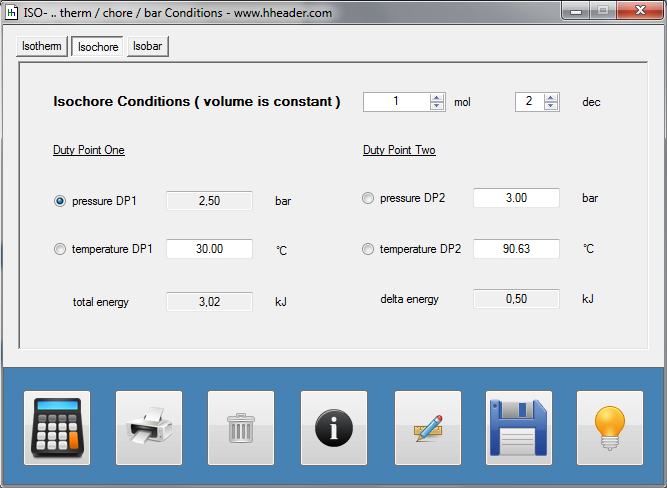
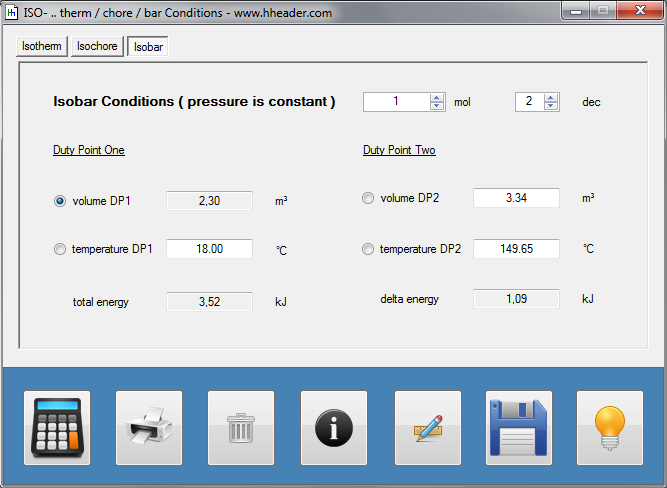
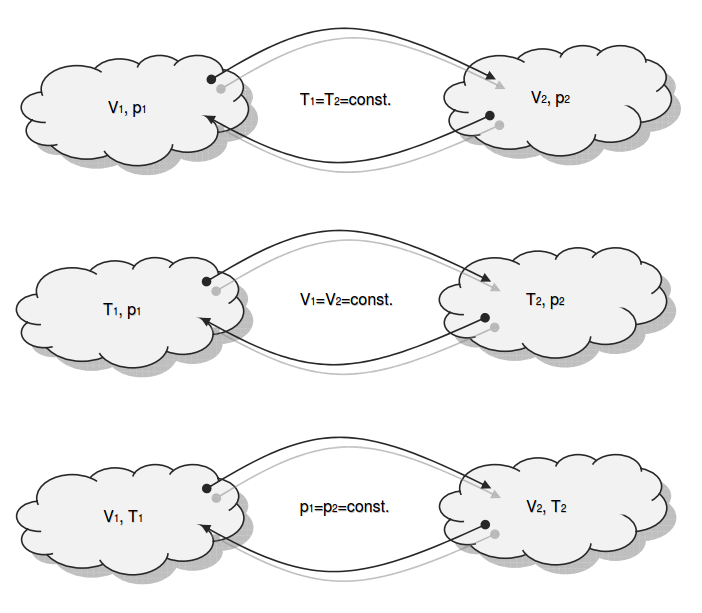
Category 4 - Energy: ISO-ConditionsBeside the specific parameters there are in general three physical values that are important for gases and which define the process circumstances significant. These are pressure, volume and temperature. They are working in coherence to each other. During the process it is often the case, that the parameters are changing slowly or that one of the parameters is constant according to the design criteria of the plant or according to a controlled process. In this case the change of the parameters can be assumed not to be isentropic or adiabatic, but it is either isotherm or isochore or isobar. |
|||||||||||||||
|
|
|||||||||||||||
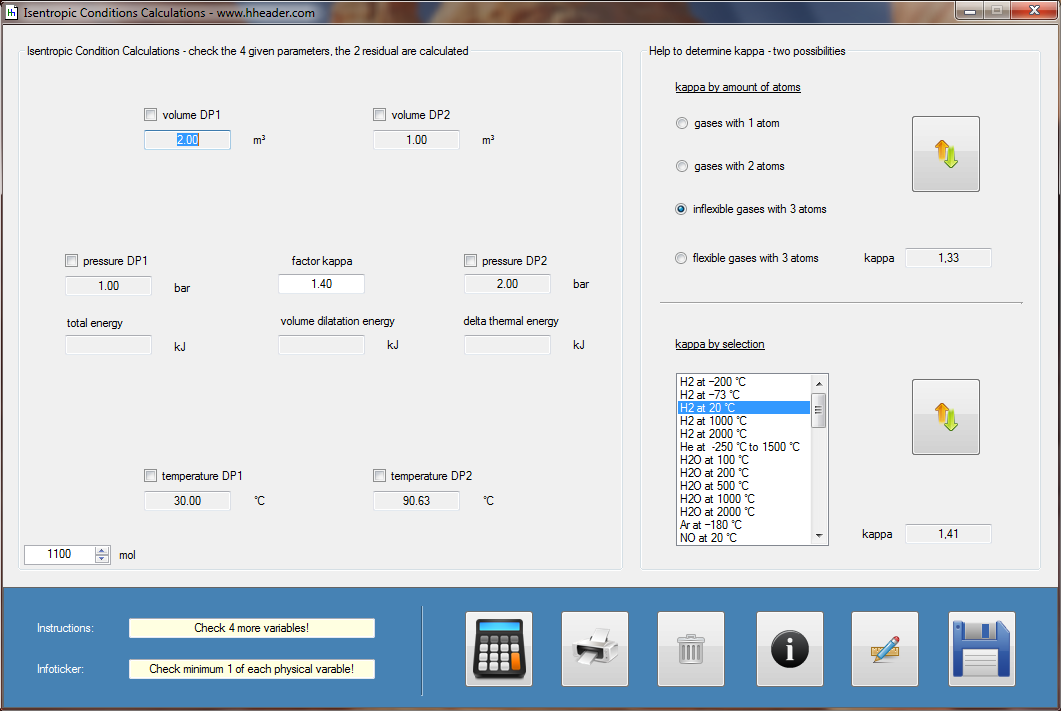
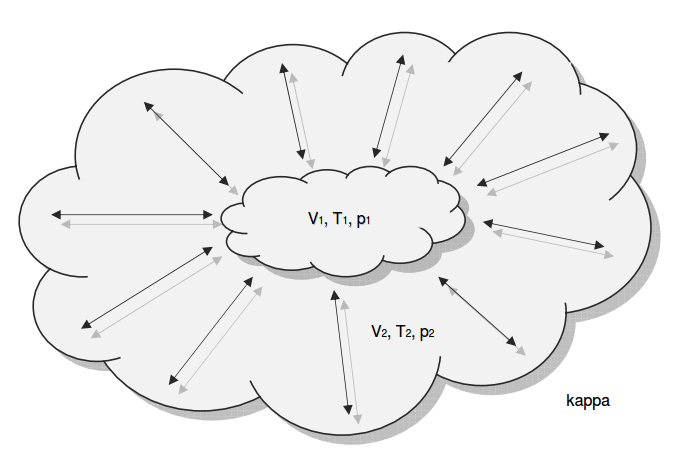
Category 4 - Energy: ISEN-ConditionsBeside the specific parameters there are in general three physical values that are important for gases and which define the process circumstances significant. These are pressure, volume and temperature. They are working in coherence to each other. During the process it is sometimes the case, that the parameters are changing rapidly or none of the parameters is constant. In this case the change of the parameters cannot be assumed to be either isotherm or isochore or isobar, but it is to be isentropic. Isentropic means that there is no heat transfer while the change of parameters and circumstances takes place. |
|||||||||||||||
|
|
|||||||||||||||
|
|||||||||||||||
|